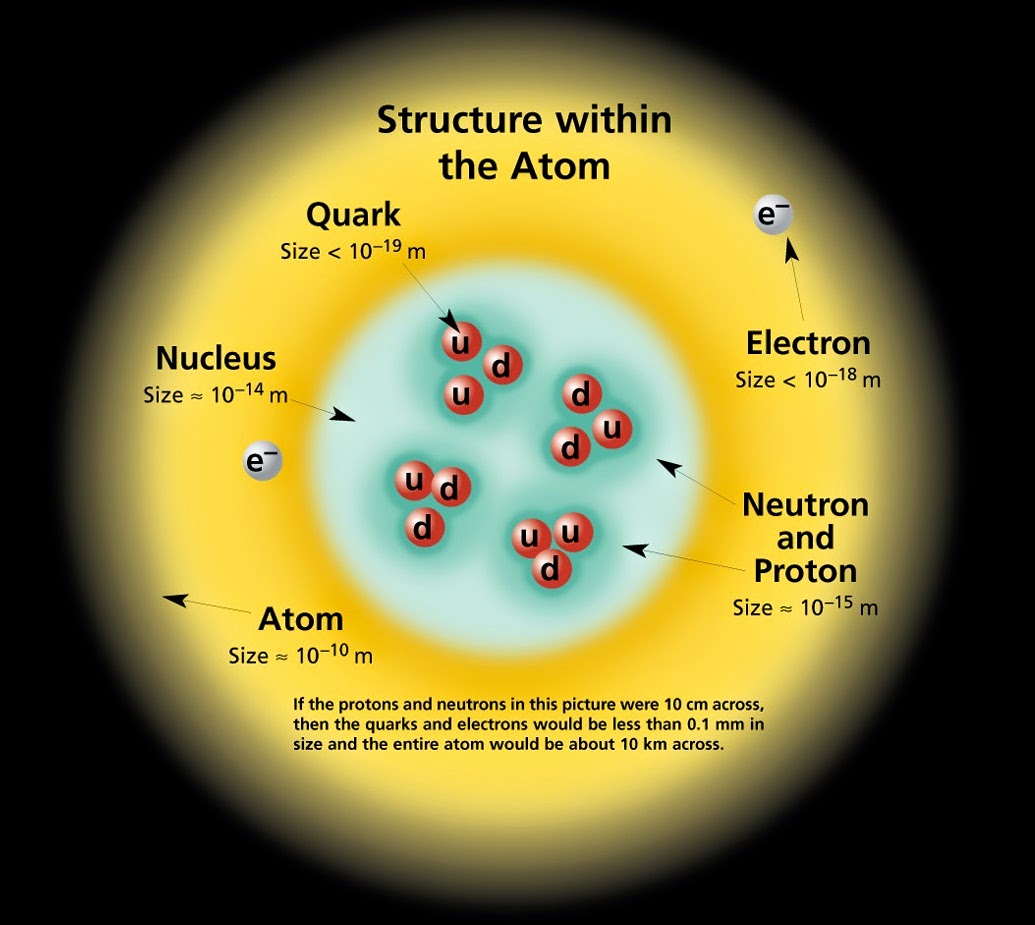
How LEDs work and a short guide on Semiconductors
Before explaining how Light Emitting Diodes (LEDs) work, let me give a quick introduction on semiconductor. Semiconductors are not good conductors of electricity due to very few free electrons (about 4-6 orders less free electrons than metals). This has to do with chemical bonding in the semiconductors, check this post . But, semiconductors can be manipulated by doping them with foreign elements to increase the number of free electrons. Let us take Silicon (Si) for example, Si has 4 covalent bonds with 4 adjacent Si atoms. If we dope Si with Phosphorous or Arsenic (P and As), we will end up with one extra free electrons as P or As can form 5 bonds. Similarly if we dope Si with either Boron or Alumnium (B and Al), we will end up with a free hole (absence of electrons, these can conduct electricity too) as B and Al can only form 3 bonds. (Source of the image: LINK ) Doping is know as n-type if it results in an extra electron and p-type if an extra hole is created. An...